Wireless pacemakers are a better fit for children—but there’s a catch
About 1 in every 15,000 children are born with congenital heart block.
These children experience an abnormally slow heart rate, which leads to lightheadedness, shortness of breath—and, in extreme cases, heart failure.
For patients battling this condition in the long term, medications may not suffice. In these cases, doctors turn to pacemakers. However, traditional wired pacemakers are not as compatible with a child’s body as with an adult’s.
A new study marks a promising development for children who need pacemakers: Wireless pacemakers offer a better fit when they can work in a pediatric patient’s body.
That word “when” is very important. Because the potential boon of wireless pacemakers for children with congenital heart block comes with a catch: The pacemakers require a catheter that isn’t currently manufactured at the right size for most children.
Why are pacemakers so challenging with pediatric patients?
The issue with installing pacemakers in pediatric patients trickle down to two simple facts about children: they grow and they move.
“The amount of physical activity that children do far exceeds a 60- or 70-year-old,” said Maully Shah, the study’s lead author and director of cardiac electrophysiology at the Children’s Hospital of Philadelphia.
Plus, over 70% of people with pacemakers are at least 65 years old—they’re not growing like children do.
“None of these leads are really tested when they’re manufactured to accommodate for growth,” Shah said.
Transcathetic Leadless Pacemakers (TLP)—or wireless pacemakers—have already been approved as a safe and effective alternative to traditional pacemakers for adults with indications for pacing. Shah’s team sought out to evaluate whether these devices could serve as a safe and effective alternative for children—for whom opting for the traditional devices might be less favorable.
Due to children’s exemption from clinical trials for devices such as pacemakers, there’s been uncertainty over the efficacy and safety of using TLPs in children. For the same reason, Shah’s team couldn’t run a clinical trial to test their hypothesis. Instead, their study was a retrospective analysis of real-world data: Physicians across 15 health centers had implanted TLPs in 63 children over a five-year period.
Where are the child-size catheters?
Almost all of the children in the study’s dataset had successful implantations, and the device remained effective in up to 10 months—and for over a year in at least a third. The researchers concluded that, at least in the short term, wireless pacemakers way be a safe and effective option for pediatric patients.
However, they also concluded that, until device manufacturers create catheters that properly fit children’s veins, the TLPs can’t be widely offered to the pediatric population.
Currently, two wireless pacemakers have received FDA approval in the U.S.—from Medtronic and Abbott. At the time of the study, only Medtronic’s Micra pacemaker was available. Shah, who is now consulting for Medtronic, said that the catheters used to implant the device are too large for children, making dangerous complications possible.
“You’re working in a very small space, which is a pediatric heart,” Shah said. “The target is very small and the equipment is very large.”
Plus, the researchers pointed out, for the Medtronic device to fit more children, it should be miniaturized even further, since the device’s size may still be too big for some, potentially causing leakiness in the heart’s right valve.
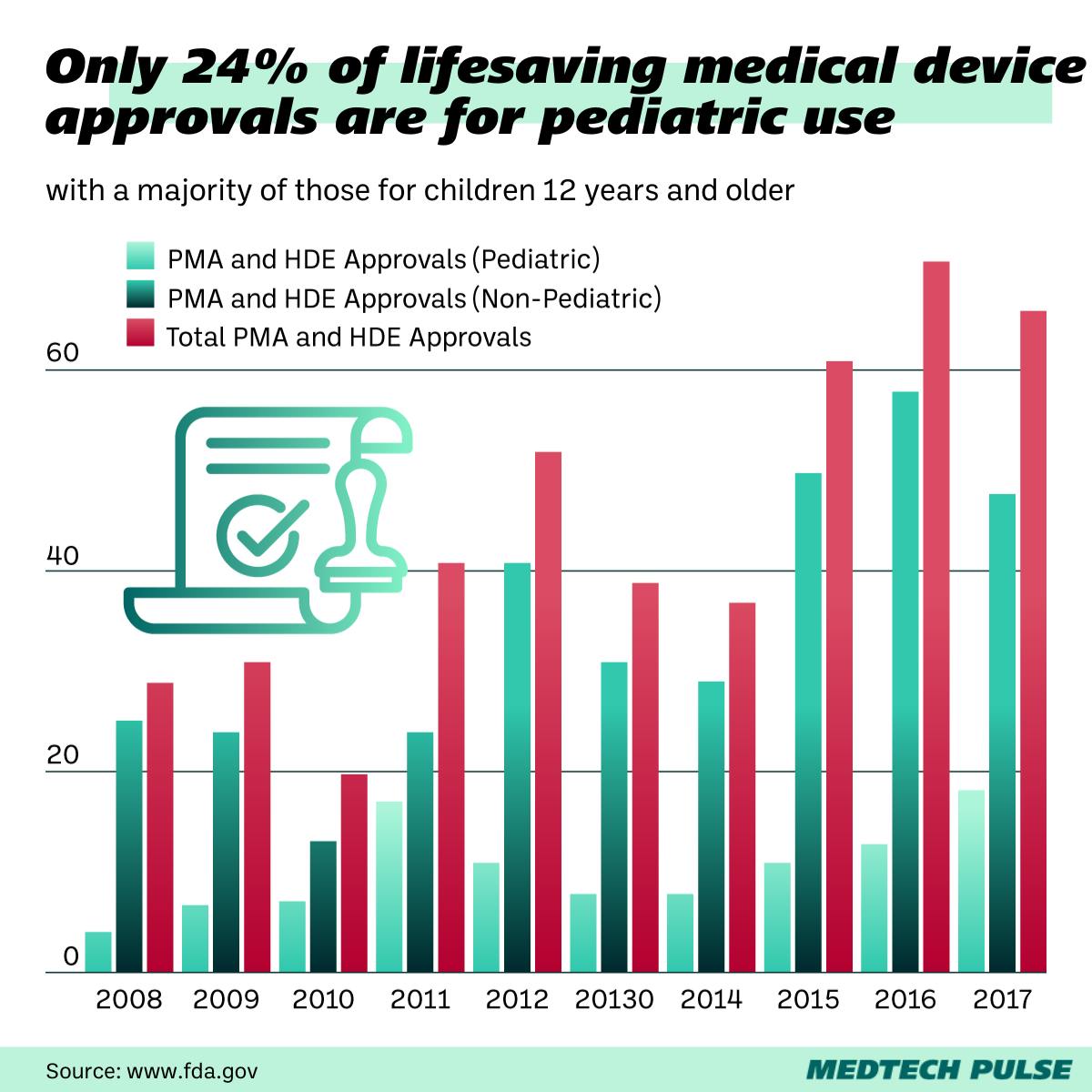
The takeaway: Pediatric medical devices can be a hard sell for the medtech industry
When laid out so plainly by researchers, the solution to this unmet need seems to be simple: device manufacturers should produce child-size catheters. And perhaps further decrease the size of the device overall.
But as with the real-world design, approval, and production of all medical devices, it’s not so simple.
Beyond pacemakers, pediatric medical devices overall are a challenge in our industry. There are two main reason for this:
- There is little financial incentive. The population of children who need medical devices like pacemakers is small when compared to adults, so the market isn’t big.
- Qualified pediatric patients are hard to enroll in the large clinical trials the FDA requires of high-risk devices such as pacemakers.
So, pediatric providers often rely on off-label use of devices solely tested in adults.
For their part, Shah and other leading cardiac electrophysiologists are working to push the FDA to invest in pediatric device development—for wireless pacemakers and beyond. Pediatric device approvals continue to lag behind the lifesaving medical devices approved by the FDA for adults.
We cheer these providers and researchers on and look forward to one day celebrating the fruits of their fight. The children who need these devices deserve it.