This non-invasive spinal cord stimulator treats paralysis
For some paralysis patients, choosing between bodily limits and invasive interventions may be a thing of the past.
Onward Medical’s non-invasive spinal cord stimulator is helping spinal cord injury (SCI) patients regain function while skipping surgery.
In a trial of 60 participants, the device’s external spine simulation paired with rehab therapy improved hand and arm function in 72% of participants. Onward submitted the trial to the FDA as part of its Breakthrough Devices Program application.
How does the stimulator work? And should we forget about brain-computer interfaces (BCIs) and invasive procedures for SCIs altogether? Let’s take a closer look.
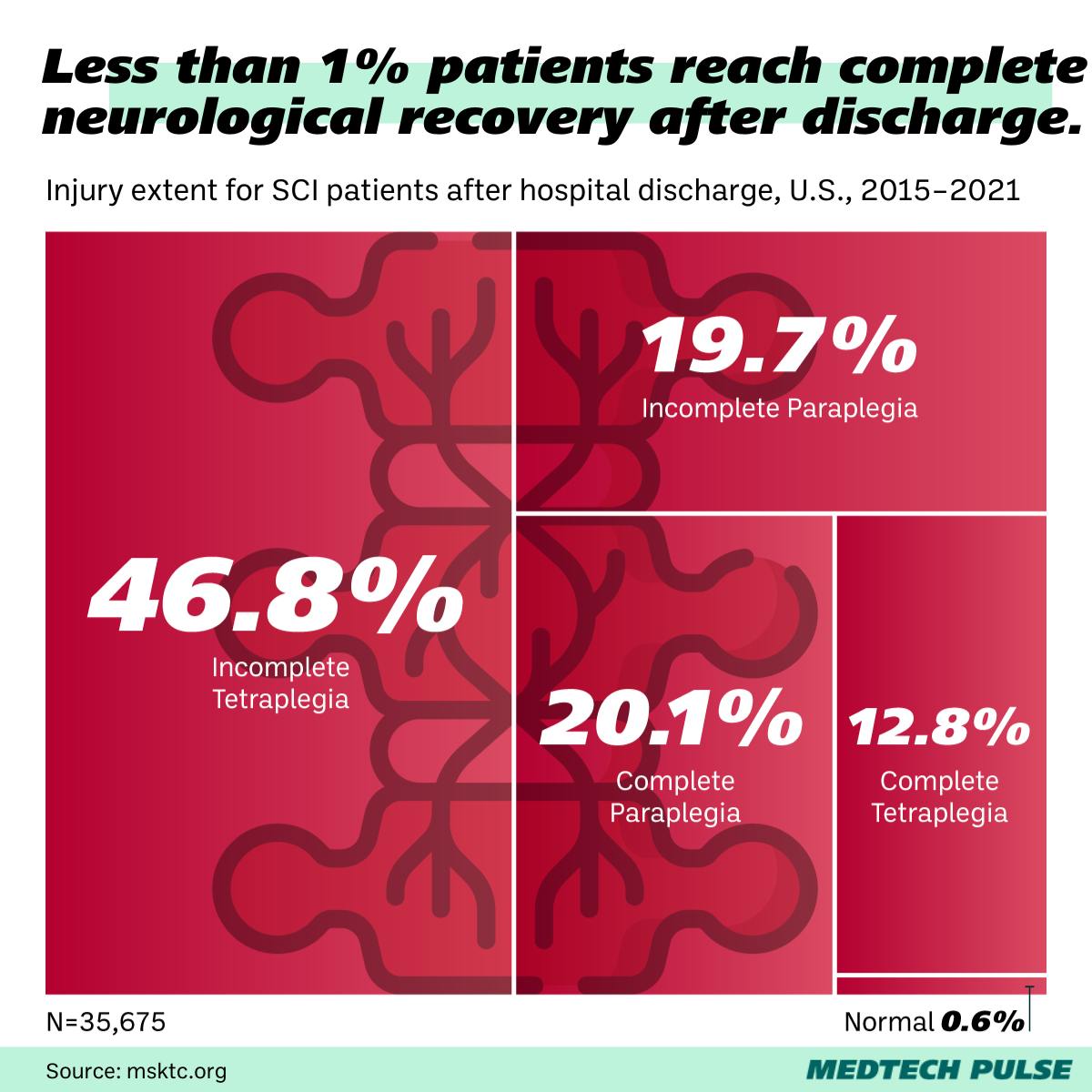
The SCI status quo
As a complicated and debilitating condition, SCIs are inevitably a darling of medical device innovators. Just in MedTech Pulse, we’ve covered interventions ranging from brain-controlled electric wheelchairs and wearable robotic exoskeletons.
Besides inspiring our collective imaginations with images of cyborgs, these innovations have the potential to dramatically improve many people’s lives. Just in the U.S., 18,000 people are estimated to suffer an SCI each year.
Historically, physical rehabilitative therapy has been patients’ only option.
Onward’s approach to tackling paralysis with stimulation piggybacks off of approaches to treating chronic pain. Research over the past few years has shown that this kind of stimulation can awaken paralyzed limbs.
Much of the current research and product development has focused on implantable stimulators. Onward itself is also developing an implantable device, but the non-invasive approach may be more exciting due to its accessibility.
Non-invasive spinal cord stimulation—why does it work?
Here’s where a key limitation of Onward’s technology comes into play. Researchers don’t quite understand how or why this approach works.
That’s why this device and treatment are not likely to help patients restore their mobility in a vacuum.
“Non-invasive spinal stimulation is very appealing. Is it a good competitor to invasive stimulation? I’m not sure,” said Dimitry Sayenko, an assistant professor of neurosurgery at Houston Methodist. “I think that non-invasive spinal stimulation, by itself, or even in combination with activity based therapy, is unlikely going to change the current status quo. It may be a step forward, but it’s definitely not the answer.”
However, to SCI patients, even the small improvements seen in patients using the non-invasive stimulator can make a massive difference.
One patient interviewed by STAT, Sherown Campbell, pointed out the following improvements in his quality of life: He could more easily wash himself and apply deodorant. His typing speed improved and he could open jars with his hands instead of his teeth. Perhaps most importantly, he could better grip a steering wheel and be a better “child Uber driver” to his two kids.
If the FDA responds positively to Onward’s application, the non-invasive stimulator would become the first such device cleared by the agency to help SCI patients restore arm function. With the lower risks and price point of a non-invasive approach, we welcome even the marginal gains this product will afford SCI patients.