Patient safety five years after a landmark medical device investigation
Do you remember the Implant Files?
Five years ago, the International Consortium of Investigative Journalists (ICIJ) conducted and began publishing a series of investigations along with 252 journalists and 59 global media partners.
The stories in the series ranged from discussions of breast implant injuries to the dangers of implant tourism.
The impact of this journalism traveled the globe, spurring important conversations, investigations, and regulations from Australia to the U.S.
Today, we’re going to take the opportunity of the fifth anniversary of the original investigations to shed light on the medical device safety changes that have happened since. We’ll also take a look at the history of big medical device safety stories and discuss the importance of available, accessible information on medical technology.
The medtech world after the Implant Files
If you know the ICIJ from anything, it’s likely from the infamous 2016 Panama Papers investigation.
But with the Implant Files, the organization made history in our industry, launching the first global investigation into the harm caused by inadequately tested medical devices.
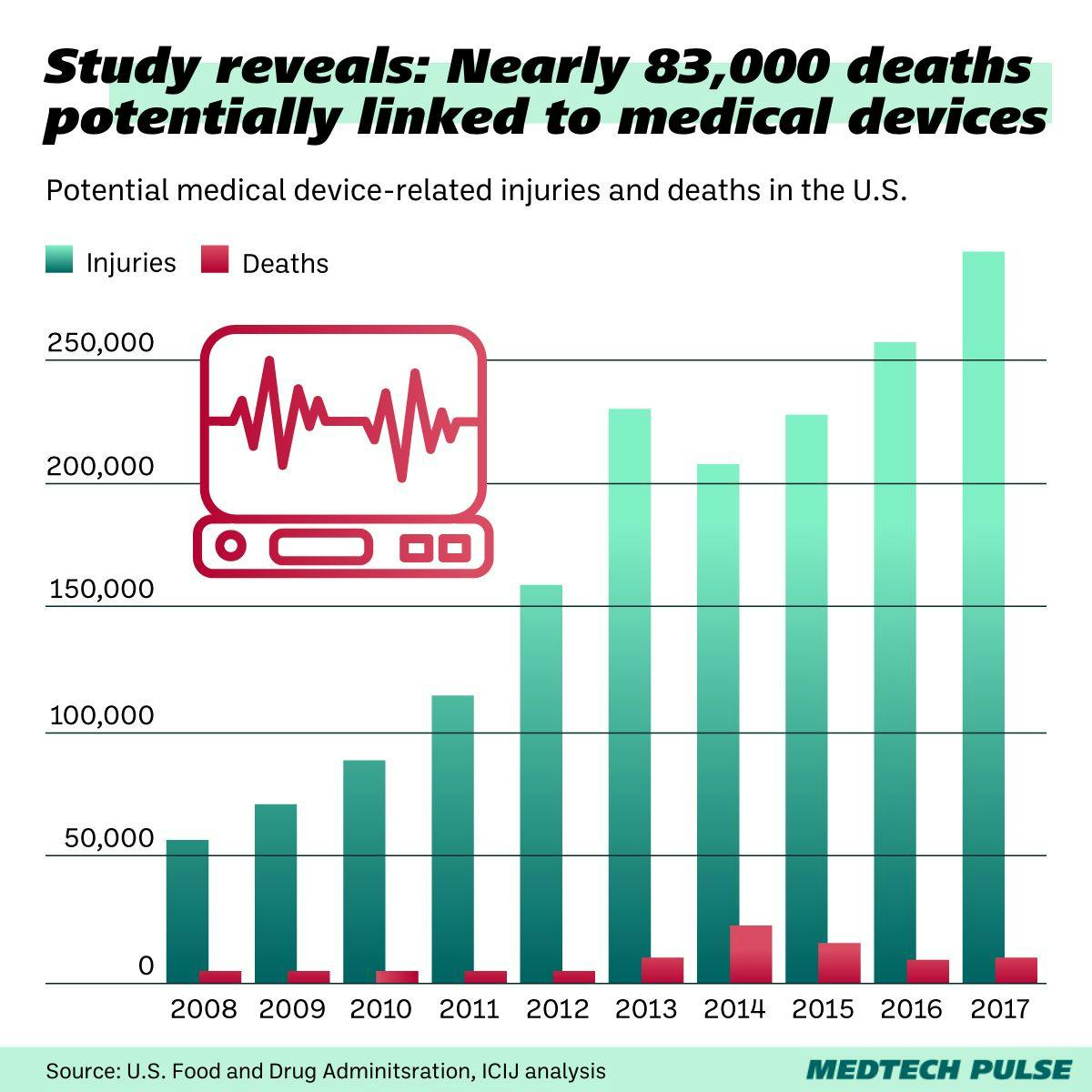
The ICIJ’s Implant Files came together over the course of two years and eventually won the organization 11 investigative journalism awards.
And for the medtech world? Their investigations directly and indirectly led to a lot of regulations we operate within today.
Since the Implant Files:
- The German health ministry created a medical device registry to improve the existing medical device approval and monitoring system.
- Australia banned 25 models of textured breast implants, which were found to be associated with an elevated risk of a rare immune cancer.
- The U.K. banned spinal implants after the Implant Files called out the devices’ minimal testing.
- India increased its medical device regulation standards.
- Canada moved towards creating a breast implant registry.
- The U.S. FDA recommended a black-box warning for breast implants.
- In September, U.S. legislators introduced the Medical Device Recall Improvement Act to mandate an electronic system—Device Events—to track recalled devices and improve notifications.
Our perspective: A history of patient advocacy
The Implant Files joined a long history of harrowing medical device-gone-wrong stories that have shaken our industry for decades.
Looking back to the 1970s, one of the first of such stories was the class action lawsuit regarding the defective and deadly Dalkon Shield IUD. The media, political, and legal storm that resulted in the shutdown of the company behind the device—and years of anti-contraceptive stigma—hearkened back to the device’s marketing. Patients were furious that they’d been promised safety and protection and instead, they were failed.
Today, that history is followed by work like the Impact Files—and even more mass-market content like Netflix’s The Bleeding Edge documentary.
Our industry may often worry about regulation slowing us down—impacting innovative companies’ viability, keeping long-requested devices from patients who need them, and risking device shortages.
But regulators and patient advocates aren’t our foes. Our industry must partner with patient advocacy groups to build trust and understand important safety concerns. Not just to avoid disasters like those on Netflix and in the Implant Files. But so that we can best serve the people we innovate for.
In the meantime, we at MedTech Pulse are going to keep bringing you stories we think are worth highlighting. Whether it’s about safety risks or fascinating innovations, we believe medtech is always worth discussing.