Dispatch from the amputation epidemic: A device that turns veins into arteries
Limb amputations are traumatic for patients.
And for many amputees, the surgery is not the end of the road—post-amputation complications and mortality abound. So, whenever amputation is preventable, it can spare patients a lot of pain and risk.
A new device from French startup LimFlow has arrived to try to prevent some of these avoidable amputations. The device uses a surgical technique to treat peripheral artery disease (PAD), stenting a blocked artery to an open vein.
This type of surgery was previously quite invasive and risky, but LimFlow’s device allows providers to do it percutaneously using a catheter inserted into the patient’s foot.
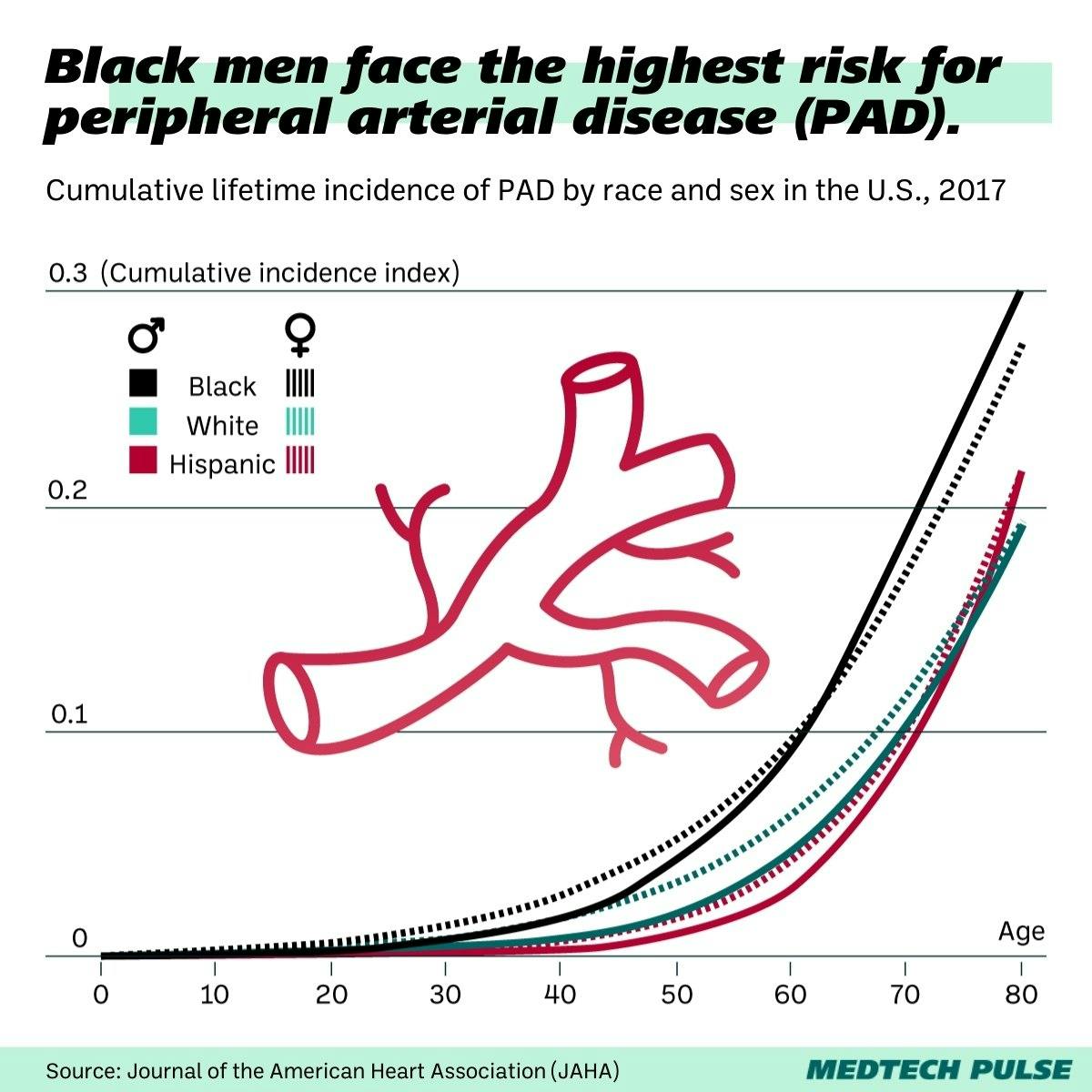
The tool can potentially help prevent many amputations, which in the US disproportionately impact Black patients. It’s a new weapon against avoidable amputations, which in the U.S. have been referred to as an epidemic.
How can LimFlow help PAD patients?
PAD is so dangerous because it blocks blood flow to the legs, putting entire limbs at risk.
LimFlow received FDA approval last month for their Percutaneous Deep Vein Arterialization System to treat patients with severe PAD who have no other treatment options. When patients progress to this advanced stage, they’re often ineligible for the other common treatments—balloons and stents.
This approval followed LimFlow’s clinical trial, where 66% of patients had survived with no amputations six months after their LimFlow procedure.
“We exist between normal interventional medicine and amputation,” said Dan Rose, CEO of LimFlow. “Our goal is to make sure the amputation never happens.”
The device isn’t a magic bullet. It would continual care down the line—especially given that trial data only extended to six months. But for the right patient, it could be life-changing and limb-saving.
Why this matters: The amputation epidemic
PAD is often underdiagnosed, but a conservative estimate says that somewhere between 6.5 million Americans aged 40+ have PAD. That incidence is disproportionately felt by Black Americans.
The condition is understood to be caused by plaque buildup in the arteries, and its severity is progressive.
The patients at the highest risk for needing an amputation are those suffering from severe PAD, otherwise known as chronic limb-threatening ischemia (CLTI), which can be fatal without intervention. Approximately 11% of PAD patients progress to this advanced stage.
Black patients disproportionally face amputations due to their PAD as well.
Whenever a device can help ameliorate a health inequality like this, we get excited. However, in the case of LimFlow that excitement is cautious, given that questions about access and long-term efficacy still abound for the new device.
As with many medical devices, ethics dictated that there be no control group—since patients at such high risk shouldn’t be deprived of a potentially effective treatment. Information researchers glean from beyond the six-month trial mark will help shed more light on efficacy.
At the same time, it’s important to keep in mind that LimFlow is unlikely to be widely available. It requires physicians to be specially trained to administer the procedure. Some cardiology experts interviewed by STAT even suggested that, in some cases, going ahead with amputation may be the safer option.
LimFlow is continuing to follow its trial participants for three years and is working on a post-market study. So, time will tell the answers to these tricky questions.
In the meantime, we’re grateful for the opportunity to shed light on this lesser-known but dangerous disease. Whether it’s LimFlow or other treatments innovators come up with in the future, we believe these patients deserve more options to help them lead long healthy lives with their limbs.