A pair of devices bring relief for bladder disorders
Patients with overactive bladder (OAB) may soon have a new way to get their lives back.
Two new devices—an ankle implant and an accompanying wearable device—have received FDA approval for treating OAB.
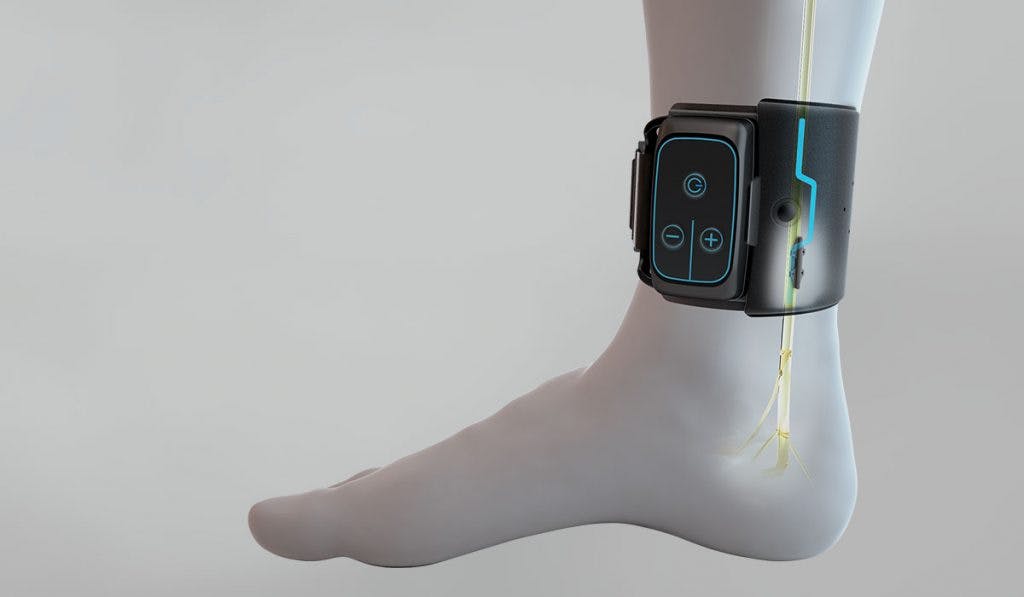
Both devices come from startup BlueWind Medical. BlueWind’s approach depends on a form of neurostimulation called tibial neuromodulation, which blocks abnormal nerve impulses around the bladder, the source of many cases of the disorder.
But here’s what’s interesting about these devices: Tibial neuromodulation is generally administered as a once-a-week, in-clinic acupuncture-like treatment, limiting its accessibility.
This device changes that.
The impact of bladder disorders
OAB is a stigmatized condition, which can lead to underdiagnosis and mask how debilitating it is.
It isn’t just occasional incontinence. Patients struggling with this condition face frequent, often uncontrollable urges to urinate throughout the day and night. It disrupts social life, work, and self-esteem. It disrupts patients’ ability to get rest and sleep through the night.
Sixteen percent of adults are estimated to struggle with OAB—and that prevalence increases with age. Because of the stigma, many patients and families treat OAB as a normal consequence of aging, not realizing there are effective treatments that can relieve their pain.
First-line treatments for OAB involve dietary and behavioral changes as well as pelvic floor therapy.
The next options are bladder medications and injections that relax the bladder. For patients experiencing OAB with menopause, vaginal estrogen can also help strengthen urethral musculature.
And then we come to neurostimulation. The most effective option is a sacral nerve stimulation device, which targets the impulses of the nerve carrying signals to the bladder. However, this procedure is invasive. It is done by placing a wire under the skin in the lower back. Another approach is the one we mentioned earlier: tibial nerve stimulation.
Bringing tibial neuromodulation home
Traditionally, tibial neuromodulation involves a thin needle placed under the skin of the ankle to send impulses through the tibial nerve to the spine, where the message then gets relayed to the bladder.
Patients come in for these treatments once a week for three whole months, and then likely need maintenance treatments every three to four weeks after that. In other words, access to the treatment is limited to a patient’s ability to come to a clinic quite frequently.
That’s where at-home neuromodulation devices come in.
“We see patients who are driving from three, four hours away,” said Alexis Dieter, a urogynecologist who is helping test a similar technology developed by Coloplast. “This really expands the options for patients, especially those who don’t have immediate access to a urogynecologist.”
BlueWind’s device requires in-office implantation with a minimally invasive procedure under local anesthesia. Then, patients place the accompanying wearable next to the ankle once or twice daily to activate the neurostimulation. The device requires no batteries and, according to BlueWind, is unlikely to require future surgery for implant adjustment.
With only a single outpatient procedure, millions of patients could have an effective treatment for their OAB. BlueWind’s trial had over 80% of patients experiencing a 50% reduction in urge incontinence episodes at 12 months. While not curing them completely, this could be a tool for many patients to get parts of their lives back—without trading in their time for endless clinic visits.