A new non-invasive test gives IVF an overdue upgrade
In vitro fertilization (IVF) is one of the hardest ways to have a child. It’s expensive, invasive, and emotionally taxing.
Yet, many people around the world have been turning to IVF and other assisted reproductive technologies (ART) since the first IVF birth in 1978. Why? According to the WHO, roughly 1 in 6 people worldwide are affected by infertility, defined as struggling to conceive for longer than one year.
Luckily, a new innovation from University of California, San Diego (UCSD) researchers may soon make IVF more efficient—and perhaps even more accessible and successful.
The innovation? A new method for testing embryo quality.
What’s the deal with embryo quality?
Embryo quality is one of the biggest bottlenecks of achieving successful pregnancy via IVF.
But before we understand why, let’s remind ourselves of the basics of IVF. Here’s a breakdown of the basic steps involved in one IVF cycle:
- Egg retrieval from the hopeful mother or an egg donor. This step is preceded by self-administered hormonal injections leading up to the retrieval, a minor surgery called follicular aspiration.
- Sperm collection from the hopeful father or a donor. Non-invasive and simple in the way you imagine.
- Lab-based (in vitro) fertilization of the egg(s) with the sperm.
- Analysis of the resulting embryo(s).
- Implanting embryos in the uterus using a catheter. Often, when possible, multiple embryos are transferred to the uterus to increase the odds that one will successfully implant. This is why multiples are common in IVF pregnancies.
After these five steps, the ideal outcome is a positive pregnancy test. If that doesn’t work out, patients can opt for another cycle.
As you can see, each cycle comes with its physical discomforts—and financial costs, with an average bill of $12,400 per cycle in the United States. And this outlined process does not even account for the blood tests and transvaginal ultrasounds women undergo for monitoring along the way.
And while Step 4 is one of the steps with the least amount of active involvement from the hopeful parent(s), it’s one of the steps with the most nervous anticipation tied to it.
Currently, the embryo analysis primarily relies on genetic testing via biopsy or morphological embryo quality assessment.
The former is effective in providing a trove of valuable chromosomal insight into embryo quality, but its invasive nature may in itself compromise embryo quality. The latter, to put it in crude terms, is a complex way of evaluating embryo quality based on how it looks.
While time-lapse imaging technology can give us valuable information about an embryo’s viability, the study authors behind this new embryo quality test point out the information we can get from morphology is limited.
“This is often a really trying experience, where we often say: ‘We don’t know why an embryo is good. We don’t know why an embryo doesn’t result in a baby.’ And we can’t know why until we better understand what’s happening in the embryo,” said research UCSD professor H. Irene Su.
Instead, this new test relies on extracellular RNA (exRNA). This is genetic material left behind in the liquid the embryos are grown in. The researchers identify exRNA profiles that differentiate between embryos growing to the blastocyst (implantation-ready) stage and those that have been arrested in growth. They then trained a machine-learning model on these profiles, helping it identify higher-quality embryos.
IVF is all about timing
Of course, this test is far from being clinic-ready.
“This model may help identify genes that are valuable in development, but I would want to see the model applied to individual embryos and it is far from being a tool for embryo specific selection,” reproductive endocrinologist Victoria Jiang said.
In other words, more research is needed before these insights can make their way to speeding along individual IVF cycles. But giving patients and providers more insight as to whether an embryo is likely to result in a successful pregnancy is likely to make IVF more efficient in the long term.
And efficiency is one of IVF’s biggest headaches.
Even putting aside the financial cost of each IVF cycle—especially in a nation like the U.S. where ART is seldom covered—time is one of fertility’s most precious resources.
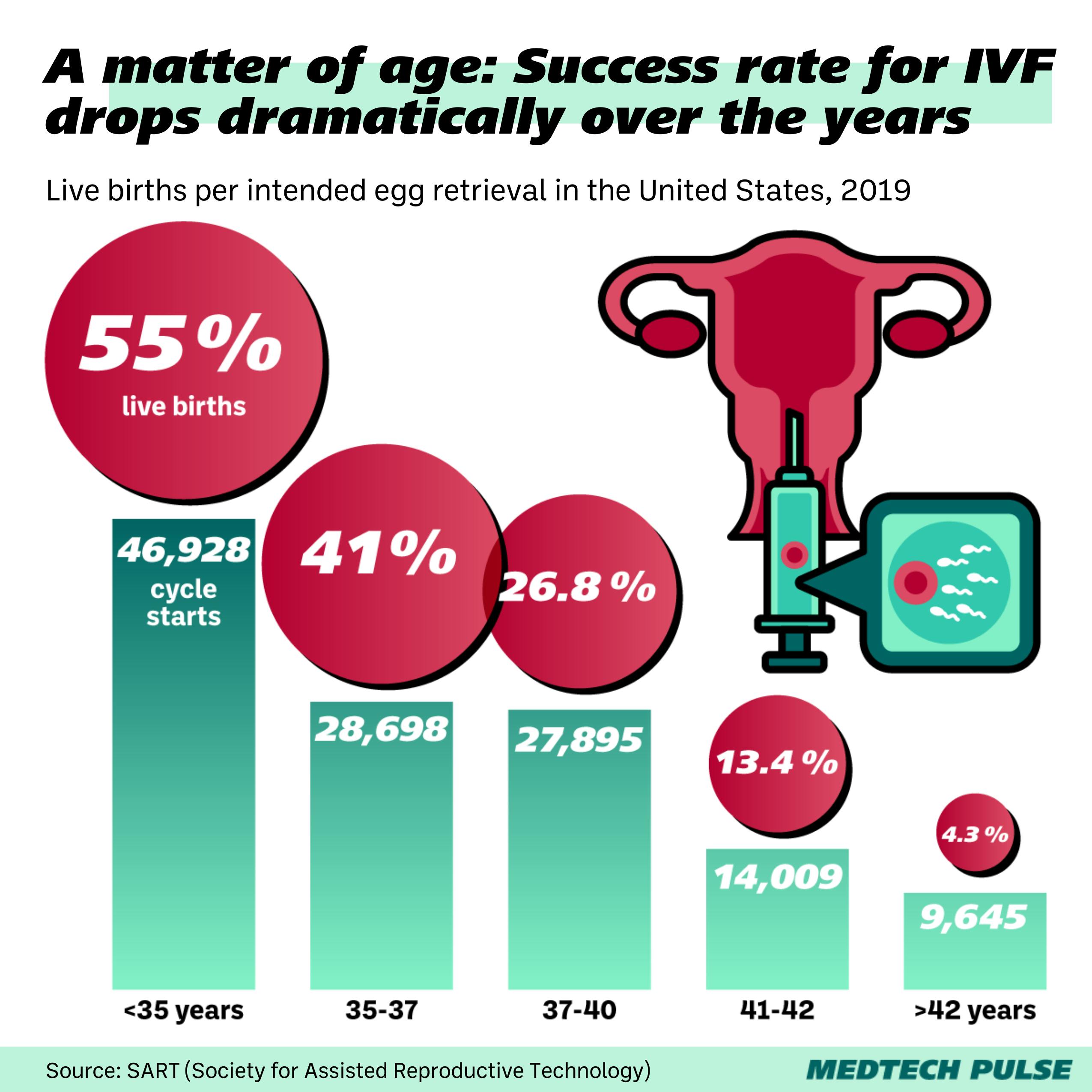
With each failed IVF cycle, patients’ odds at achieving successful pregnancy and live birth lower. For women under 35, the odds rest at just over 50% of having a live birth after one IVF cycle. Those odds drop to 25% for women age 38+.
With this research, clinicians and patients can feel hope for saving a bit more time in the IVF process, ultimately increasing the odds that the process can give them what they most want: healthy births.